- Frameworks
- ISO 13485
ISO 13485 preparation & management made easy
Easily prepare for and maintain ISO 13485 certification – the gold standard for a quality management system (QMS) in the medical device industry. Leverage Strike Graph’s powerful tools and automated workflows to confirm readiness for your first ISO 13485 audit through ongoing post-market surveillance.
Ready to see Strike Graph in action?
Find out why Strike Graph is the right choice for your organization. What can you expect?
- Brief conversation to discuss your compliance goals and how your team currently tracks security operations
- Live demo of our platform, tailored to the way you work
- All your questions answered to make sure you have all the information you need
- No commitment whatsoever
We look forward to helping you with your compliance needs!
Find out why Strike Graph is the right choice for your organization. What can you expect?
- Brief conversation to discuss your compliance goals and how your team currently tracks security operations
- Live demo of our platform, tailored to the way you work
- All your questions answered to make sure you have all the information you need
- No commitment whatsoever
We look forward to helping you with your compliance needs!
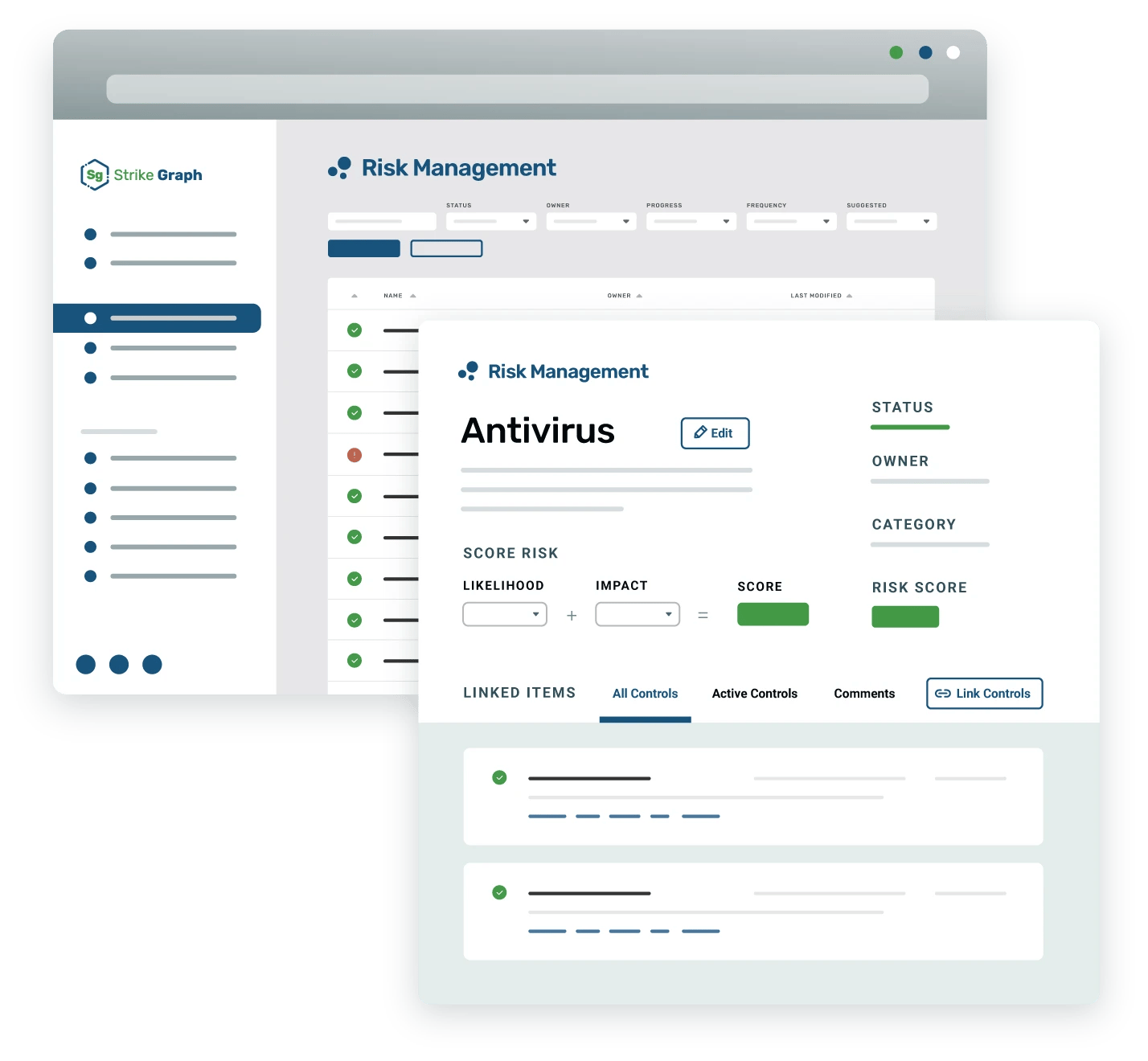
Your complete solution for ISO 13485 compliance and beyond
With Strike Graph, customers enjoy a comprehensive solution where they can manage audits, maintain compliance, and integrate additional frameworks with ease.
Experience a solution that is tailored to the needs of medical device organizations.
We've taken the ISO13485 framework and digitized it, allowing customers to have a single source of truth as they prepare for their ISO13485 audit and then maintain compliance through post-market surveillance activities. Additionally, our custom SaMD solution customers can combine the ISO13485 framework with our SaMD framework and SBOM functionality to have a full product and cyber security compliant posture management experience.

Efficiently work towards multiple frameworks in a single platform
Unique to Strike Graph, you’ll be able to confidently meet and exceed FDA and EU regulatory standards in a single platform. For customers looking to implement additional frameworks such as SOC 2 or GDPR, you’ll be able to easily leverage your current controls to help meet other framework standards.

Always be audit-ready
Strike Graph has cyber security risk management built into the platform so you can leverage risk-based tools on day 1 and beyond. Not only can we help you get ISO 13485 certification faster, but we can also help your organization monitor controls and keep evidence up to date, so you feel confident going into future reviews and audits.
How it works:
See how Strike Graph gets you ready for ISO 13485 certification in 4 easy steps.
Design
Operate
Measure
Certify
Key features of the Strike Graph platform
The Strike Graph platform was designed to be customized to meet your unique business needs, giving you the flexibility and support to hit the ground running towards your compliance goals.
Customizations
Every medical device manufacturer has their own particular security needs and risk profiles. Instead of forcing customers to conform to a generic, one-size-fits all platform, Strike Graph enables customers to create and design their own compliance program that truly fits their needs.
Easy integrations
Strike Graph can integrate with your existing eQMS and regulatory information systems to help reduce the complexity of managing your ISO13485 technical file. Strike Graph’s seamless integrations can even automate evidence collection from over 5,000 data points within your tech stack.
Comprehensive support and expertise
Strike Graph has partnered with some of the most respected consultants in the industry to to help our medical device manufacturers at every stage of ISO 13485 certification.
Dashboards & Reporting
Strike Graph’s intuitive dashboards and robust reporting tools offer insight into your medical device security posture and help build trust with stakeholders. Assess active risks, evaluate control effectiveness, and monitor evidence status – all from a single platform.
GAP Analysis
Identify discrepancies between your current security posture and desired compliance standards. By pinpointing areas of non-compliance, we equip organizations with the ability to prioritize remediation efforts and allocate resources effectively.
Verify AI
Exclusive to Strike Graph, utilize Verify AI to validate whether your QMS documentation meets and maintains the ISO 13485 standard.
How to get certified without an expensive auditing firm.
The old way to compliance certification is a flawed and fought with human errors. Understand the ins and outs of how compliance and auditing is change for the better with technology.

Highly Recommended
"Their reporting and monitoring features let us keep a close eye on our compliance efforts, spot any hurdles, and measure how far we've come. It's been a real game-changer for managing our compliance projects"
"I have been thrilled with the progress and process of interacting with Strike Graph as a whole"
“The most helpful aspect of Strike Graph is its ability to automate compliance processes and provide clear, actionable insights. It saves our team a significant amount of time and effort, allowing us to focus on other critical tasks. The customer support is also excellent, providing prompt and effective assistance whenever needed."
Your questions about ISO 13485 answered
What is ISO 13485?
- ISO 13485 is an internationally recognized standard for quality management systems (QMS) in the medical device industry. It focuses on ensuring product safety and regulatory compliance throughout the entire lifecycle of medical devices, including design, development, production, installation, and servicing. The standard requires organizations to maintain thorough documentation and implement effective processes to manage risks related to products and their supporting systems.
Who needs it?
- ISO 13485 is essential for many organizations within the medical device industry, including medical device manufacturers, designers of medical devices, and companies that provide parts to those organizations. It also applies to suppliers of raw materials and businesses that offer sterilization, packaging, or labeling services. Additionally, organizations that install or service medical devices, as well as distributors and importers, need to follow this standard to ensure safety and quality.
What are the benefits of ISO 13485 certification?
Achieving ISO 13485 certification can help improve product quality, enhance regulatory compliance, increase market access, and build trust with customers and stakeholders.
What is the path to certification?
Key steps include planning, implementing the QMS, conducting internal audits, applying to a certification body, and undergoing Stage 1 and Stage 2 audits.
How can an organization ensure continuous compliance with ISO 13485?
The Strike Graph platform will continue to monitor controls and evidence to ensure everything stays up to date and that risks are managed and mitigated quickly.
What is the difference between ISO 13485 and ISO 9001?
- While ISO 13485 is based on ISO 9001 (the global standard for quality management), ISO 13485 focuses on specific metrics for medical device quality management performance and ensuring that your company’s QMS stays effective.
Can’t find the answer you’re looking for? Contact our team!
Start your ISO 13485 journey today
Schedule time with our medical device compliance experts to explore how we can assist you with starting your ISO 13485 journey.
Additional ISO 13485 solution resources
Our extensive library of resources will answer all your questions.
.jpg?width=1448&height=726&name=Screen%20Shot%202023-02-09%20at%202.57.5-min%20(1).jpg)







